ATOMIC LAYER DEPOSITION OF A NANOMETER-THICK Li3PO4 PROTECTIVE LAYER ON LiNI0.5MN1.5O4 FILMS: DREAM OR REALITY FOR LONG-TERM CYCLING?
SCIENTIFIC CASE

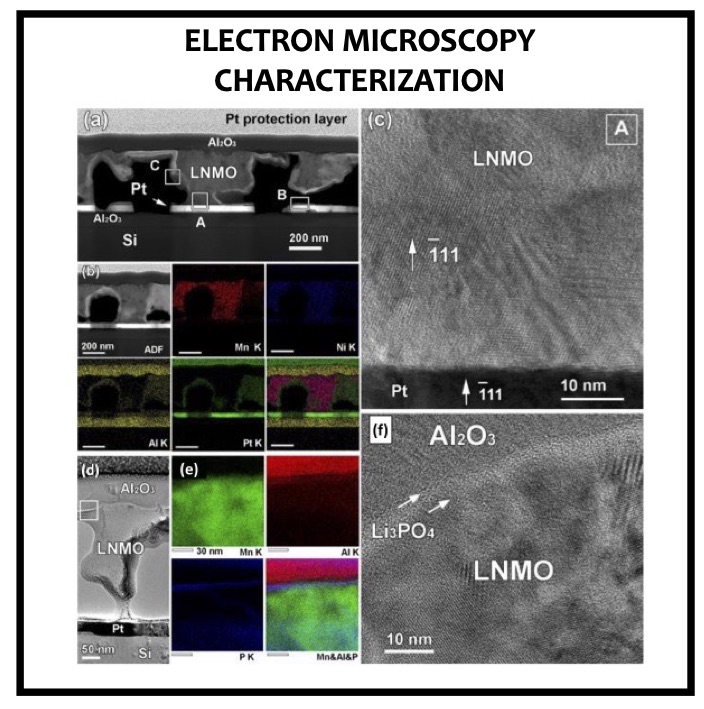
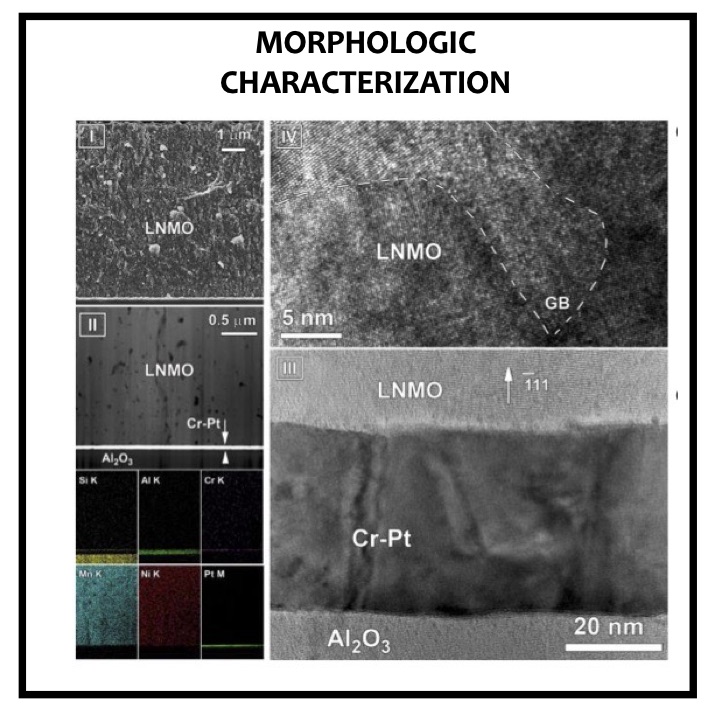
LiNi0.5Mn1.5O4 (LNMO) spinel material is considered as one of the promising electrode for lithium-ion batteries and microbatteries applications owing to its high operating + potential (∼5 V vs Li/Li+ ), high theoretical capacity (∼147 mAh g−1), and good rate capability. Curiously, one of the main technological barriers inhibiting its commercialization is precisely its high operating potential, which is beyond the electrochemical stability windows of organic electrolytes. The oxidative decomposition of the organic electrolyte (solvent molecule, lithium salts) occurring at a high potential (>4.5 V vs Li/Li+) as well as the leaching of the Mn2+ transition-metal ion from the LNMO material decrease the cycling performance. To prevent the electrolyte decomposition and/or the cation dissolution effects, the interface between LNMO spinel material and organic electrolyte has to be modified. Two strategies are followed by many research groups to fulfill those requirements. On the one hand, the use of additional electrolyte additives undergoing sacrificial oxidation without modifying the proper- ties of the main electrolyte is proposed to improve the cycling stability. On the other hand, modification of the solid−liquid interface is another solution with the deposition of a metal oxide or a lithium-ion conductor film acting as a protective or artificial solid−electrolyte interface layer (SEI).
Thick composite electrodes (>100 μm) are generally prepared by coating a slurry of micro- or nano-sized LNMO particles (active material), binding additives (e.g., PVDF), and conductive agents (e.g., carbon, boosting the electrical conductivity) on aluminum foil. Nevertheless, this preparation method does not allow studying the intrinsic properties of active materials because the additives and the conducting agent modify their electrochemical performance. An elegant solution to study the intrinsic properties of active LNMO material without any binders consists in depositing thin films on substrates. Physical vapor deposition (PVD) techniques such as pulsed laser deposition (PLD) or magnetron sputtering (MS) are widely studied for the deposition of LNMO films acting as model materials.
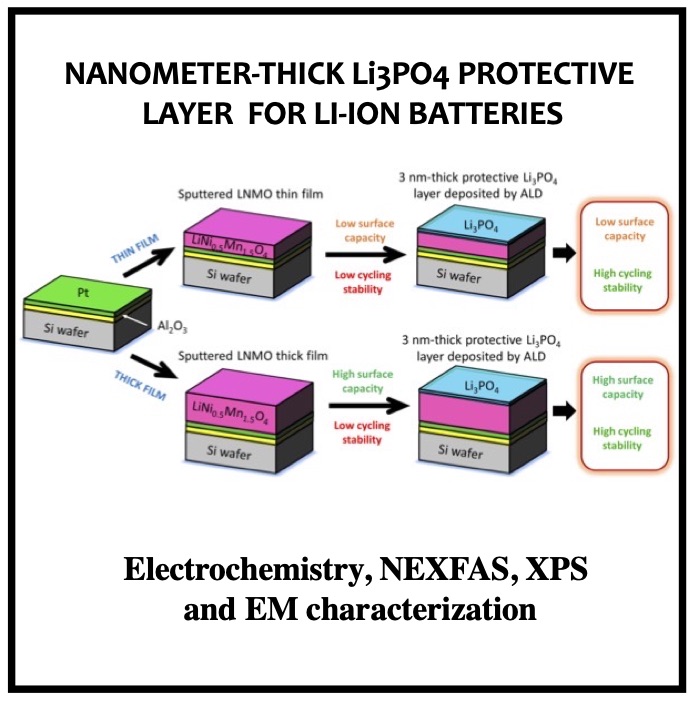
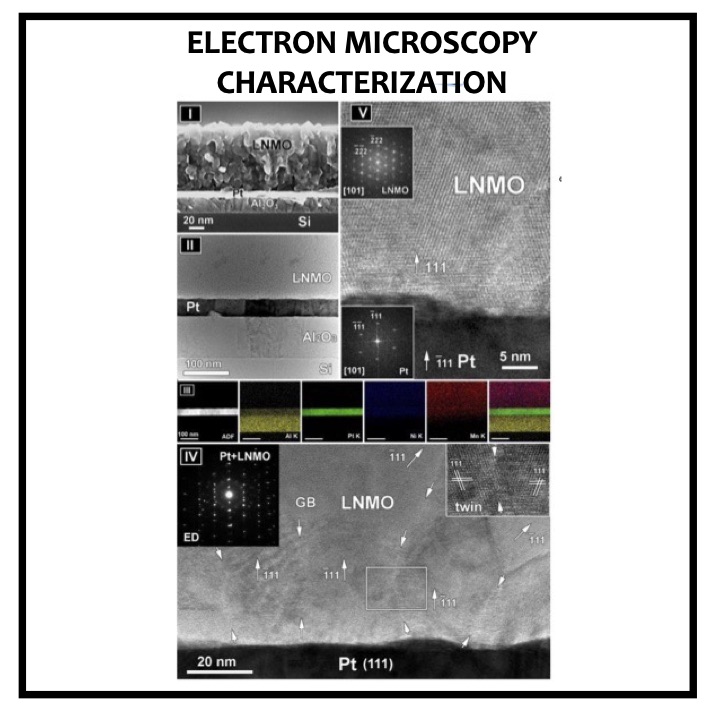
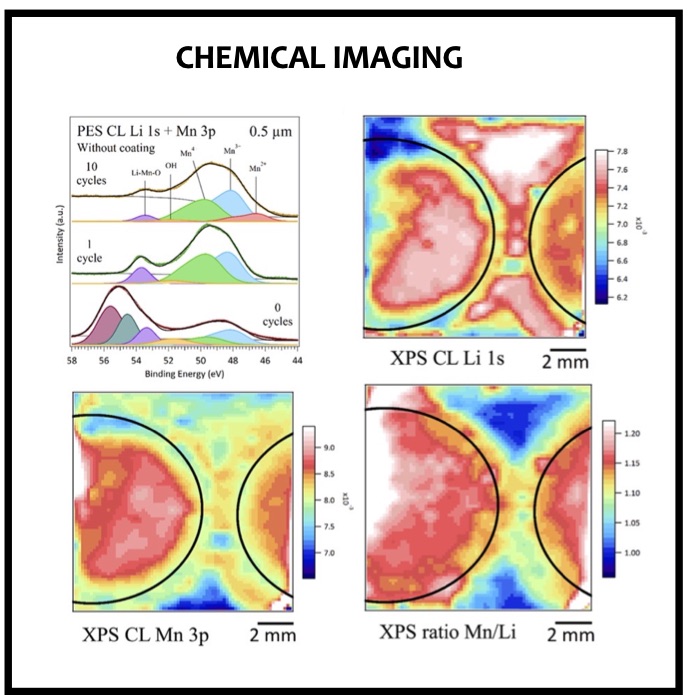
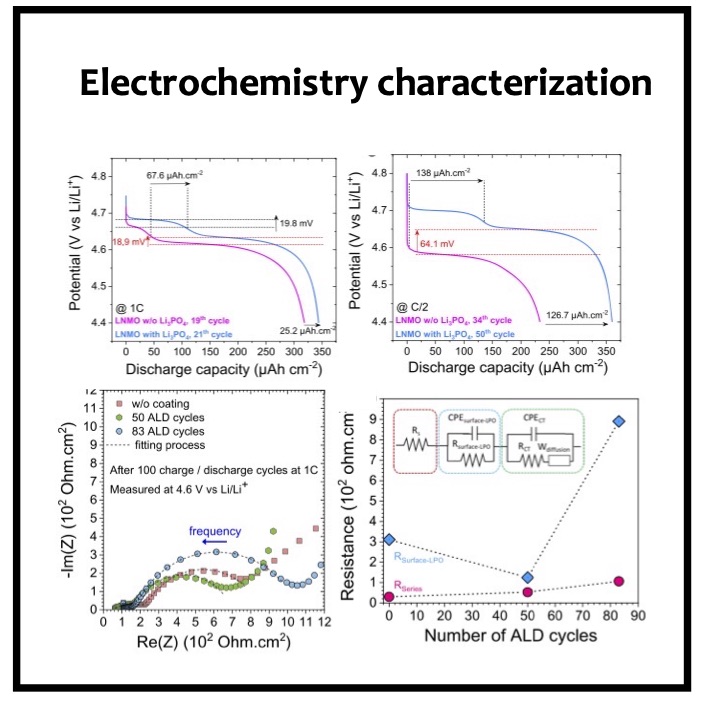
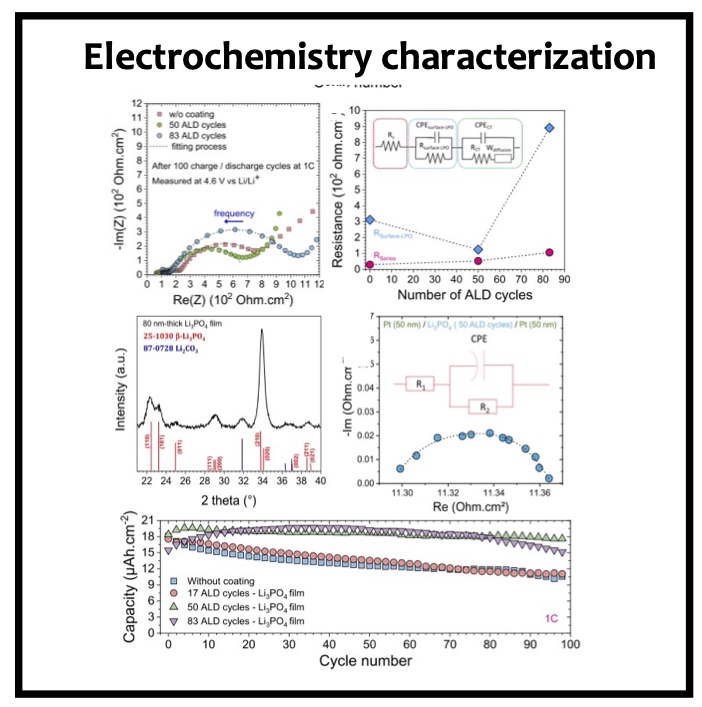
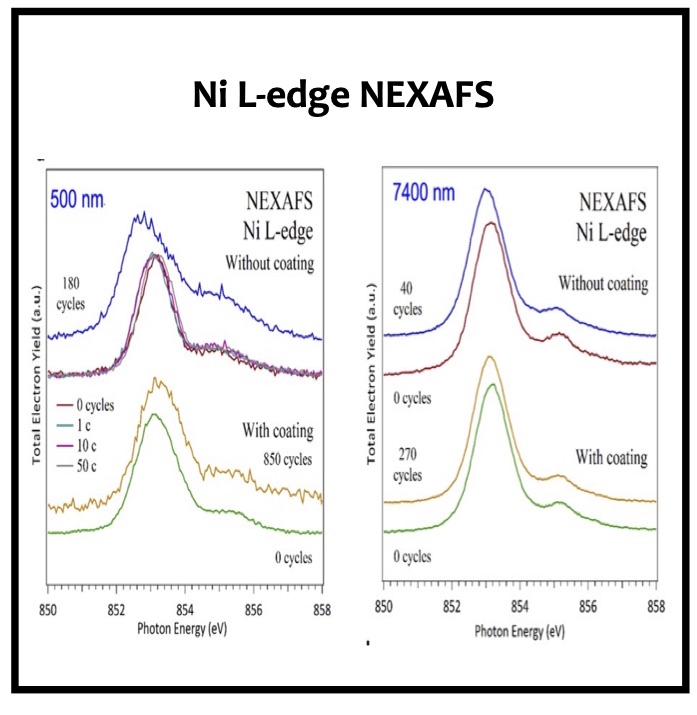
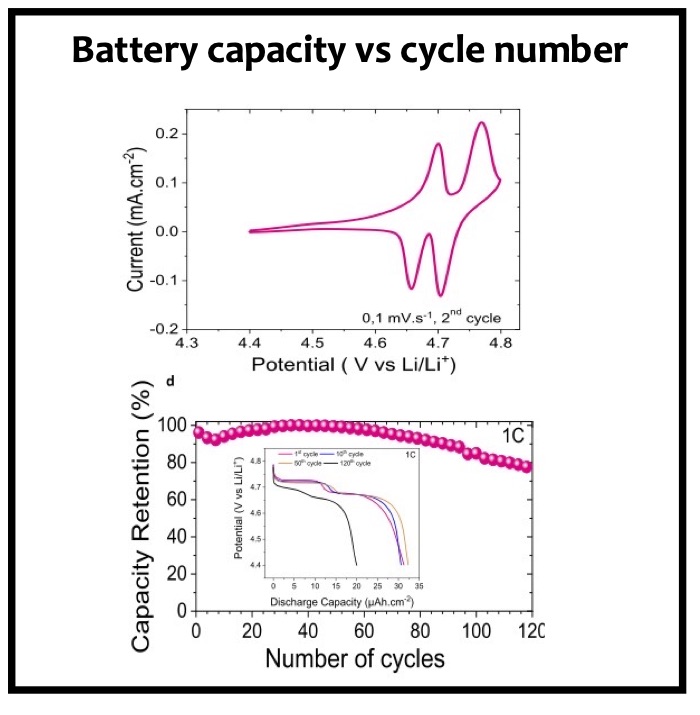
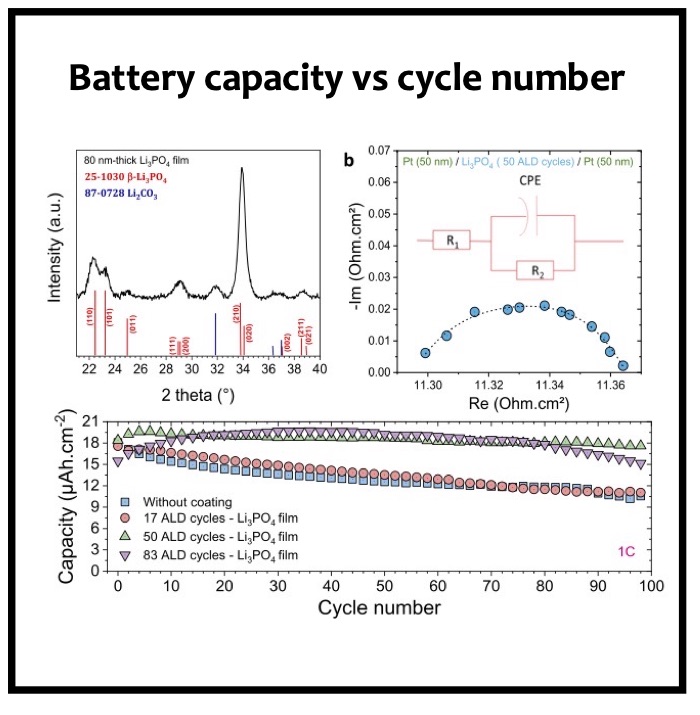
In this article we show the improvement of the surface capacity of sputtered LiNi0.5Mn1.5O4 films while enhancing the cycling stability with the deposition of a surface protective layer. We control the interfacial chemistry between sputtered LNMO films and liquid electrolyte with an ultrathin lithium-ion conducting layer coating deposited by ALD. We clearly investigate the role of the surface protective layer and demonstrate how we improve the cycling performance of high-voltage sputtered LNMO films for 5 V Li-ion batteries application. The surface capacity of 500 nm thick LNMO film is about 25 ± 5 μAh cm−2 at 1C. Without any protective nanometer-thick layer, the capacity of 500 nm thick film quickly fades after 150 cycles at this cycling rate. The cycling stability performance is pushed back toward more than 850 cycles at 1C thanks to nanometer-thick Li3PO4 layer (<5 nm) deposited by ALD on the top of the sputtered 5 V electrode, demonstrating an increase of the cycling life by a factor of 6.5 (650%). This layer prevents the undesired surface reaction of LNMO films acting as a surface protective layer but we show that it does not prevent from mechanical degradation of the film upon charge/discharge cycling. State-of-the-art synchrotron- based techniques such as electron yield near-edge X-ray absorption fine structure (NEXAFS) spectroscopy and low- photon-energy photoelectron spectroscopy or synchrotron photoemission (PES) were used to study extreme surface qualitative and quantitative chemical analyses of sputtered LNMO films as a function of film thickness. To significantly improve the surface capacity, we demonstrate that 7400 nm thick sputtered LNMO film delivers more than 0.4 mAh cm−2 at 1C. While only 40 charge/discharge cycles are achieved with the thicker LNMO electrode without any coating layer, the cycling performance is improved up to 240 cycles thanks to the protective nanocoating layer, validating the 6.5 enhancement factor.
AUTHORS: M. Hallot, B. Caja-Munoz, C. Leviel, O.I. Lebedev, R. Retoux, J. Avila, P. Roussel, M.C. Asensio, C. Lethien
COLLABORATION BETWEEN THE FOLLOWING INSTITUTIONS
-
-
Institut d’Electronique, de Microélectronique et de Nanotechnologies, Université de Lille, CNRS, Centrale Lille, Université Polytechnique Hauts-de-France, UMR 8520-IEMN,F-59000Lille,France;RéseausurleStockage Electrochimique de l’Energie (RS2E), CNRS FR 3459, 80039 Amiens, France
-
-
-
Synchrotron SOLEIL, L’Orme des Merisiers, Saint Aubin-BP 48, 91192 Gif sur Yvette Cedex,
-
-
-
Institut d’Electronique, de Microélectronique et de Nanotechnologies, Université de Lille, CNRS, Centrale Lille, Université Polytechnique Hauts-de-France, UMR 8520-IEMN, F-59000 Lille, France; Réseau sur le Stockage Electrochimique de l’Energie (RS2E), CNRS FR 3459, 80039 Amiens, France; Unité de Catalyse et de Chimie du Solide (UCCS), Université de Lille, CNRS, Centrale Lille, Université d’Artois, UMR 8181-UCCS, F-59000 Lille, France
-
-
- Laboratoire CRISMAT, UMR6508, CNRS- ENSIACEN, 14050 Caen, France
-
-
Unité de Catalyse et de Chimie du Solide (UCCS), Université de Lille, CNRS, Centrale Lille, Université d’Artois, UMR 8181-UCCS, F-59000 Lille, France;
-
Materials Science Institute of Madrid (ICMM), Spanish Scientific Research Council (CSIC), Valencia Institute of Materials Science (ICMUV), MATINEE: CSIC Associated Unit-(ICMM-ICMUV Valencia University), E-28049 Cantoblanco, Madrid, Spain;
-
-
- Institut d’Electronique, de Microélectronique et de Nanotechnologies, Université de Lille, CNRS, Centrale Lille, Université Polytechnique Hauts-de- France, UMR 8520-IEMN, F-59000 Lille, France; Réseau sur le Stockage Electrochimique de l’Energie (RS2E), CNRS FR 3459, 80039 Amiens, France;
CITATION:
Atomic Layer Deposition of a Nanometer-Thick Li3PO4 Protective Layer on LiNi0.5 Mn1.5O4 Films: Dream or Reality for Long-Term Cycling?, ACS Appl. Mater. Interfaces. (2021) acsami.0c21961. https://doi.org/10.1021/acsami.0c21961.

