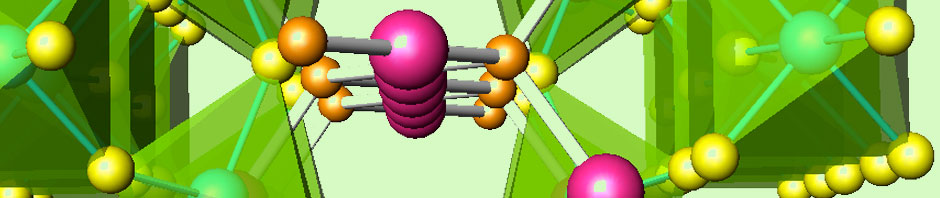
We follow three approaches for the preparation of metal hydrides. The former consists of the previous preparation of intermetallic alloys in an arc furnace.
The mixture of metals, in powder, lumps of flakes form, are pelletized and put in a water-cooled copper crucible. After evacuation of air and under high-purity Ar flow, the arc from a W tip melts the metals, the reaction takes place for several seconds to minutes; then the sample is quenched to room temperature and ground to a fine powder. These intermetallic alloys, once formed, are hydrogenated under high H2 pressure in an autoclave.

The second approach consist in the direct reaction of simple hydrides and/or metals (for instance, 2 MgH2+Ni) under hydrostatic pressure conditions, in our piston-cylinder HP instrument.
Mixtures of the reactants are introduced in gold or platinum capsules, working in a globe box. After sealing the capsules, the reaction takes place in the piston-cylinder press, at moderate reaction temperatures (typically around 600-800ºC) and for generally short reaction times (typically between 20 min. and 1 hour). Finally, the products are quenched under pressure and the capsule open, again, in a globe box.
When it is desirable to increase the amount of H in the hydride, the reactants are mixed up with NaBH4, which decomposes at the working temperature supplying an extra H2 pressure (similar to the addition of KClO4 in the case of the oxides). The high-pressure synthesis procedure offers the additional advantage of obtaining extremely well crystallized powders, which is important for the subsequent crystallographic characterization of the hydrides (most of the times by neutron diffraction)

The third approach is the mechanochemical reaction of mixtures of hydrides and metals in inert atmosphere in a ; this gives rise to poorly crystallized powders which, in this case, present the advantage of the easiness of hydrogenation-rehydrogenation.
The hydride samples are finally characterized by thermal analysis (TG and DSC techniques). A DSC1 Metteler system, able to work under 70 bar H2, has been recently set up in our Lab.