The utilization of hydrogen as energy vector in the coming decades has boosted the research and improvement of hydrogen storage procedures. New classes of materials composed of much lighter constituents (Li, Be, B, C, N, O, Na, Mg, Al, Si, P, S) have shown a much higher hydrogen storage capacity per weight than the conventional LaNi5 materials.
Especially interesting are the Mg-based hydrides, given its high mass-storage capacity; as a drawback MgH2 is thermally stable, with decomposition temperatures above 450ºC, which prevents applications. The de-stabilization of MgH2 by doping with different metals has been an active research field in the last years.
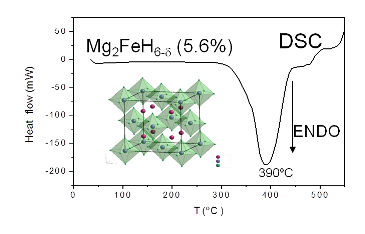
Recently we have been successful in preparing, for the first time, Mg2FeH6-d and other Mg2FeHx (M= Co, Ni) phases by direct reaction between the simple hydride MgH2 and the transition metals under high pressure conditions, in gold capsules at 2 GPa.
It is worth mentioning that Mg2FeH6 has one of the best H mass capacity ever described, almost 6%. These Mg2MHx phases are usually obtained by mechano-chemical activation, by ball-milling the precursor materials under H2, which results is poorly crystallized samples.
In our case, we obtained Mg2MH6-d with an excellent crystallinity, which would allow us carrying out a structural study by neutron diffraction and establishing invaluable structure-properties relationships.
This success in the preparative protocol has stimulated the design of a new line of research, based upon the direct reaction of simple hydrides under high pressure conditions, which prevent the thermal decomposition of the reactants. Among them we undertook the preparation of new hydride perovskites, of formula ABH3, or double perovskites, A2BB’H6, by reaction under pressure of AH and BH2.
This synthesis procedure has been rarely explored. In all these compounds the localization of H atoms, the study of the tilting of the BH6 octahedra, the presence of H vacancies etc. is also a key knowledge to interpret the sorption/desorption kinetics. RMN and neutron diffraction techniques are powerful (and unique) tools to localize hydrogen in condensed matter, which we have successfully used in a number of metal hydrides.
Recent Publications
High-pressure synthesis of Mg2FeH6 complex hydride, Retuerto, M, Sanchez-Benitez, J Rodriguez-Canas, E, Serafini, D, Alonso, JA, INTERNATIONAL JOURNAL OF HYDROGEN ENERGY 35, 7835-7841 (2010)
Crystal structure and bond valence of CaH2 from neutron powder diffraction data, Alonso, JA Retuerto, M, Sanchez-Benitez, J, Fernandez-Diaz, MT, ZEITSCHRIFT FUR KRISTALLOGRAPHIE 225, 225- 229 (2010).
Deuteration properties of CaNi(5-x)Cu(x) system, Retuerto, M.; Sanchez-Benitez, J.; Alonso, J. A.; Leardini, F.; Ares, J. R.; Fernandez, J. F.; Sanchez, C., JOURNAL OF POWER SOURCES 196, 4342-4346 (2011)