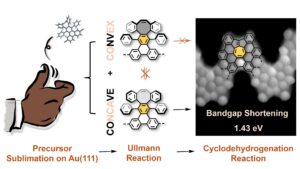
 The role of stereochemistry—the spatial arrangement of atoms within molecules—on the reaction pathway has been largely overlooked in on-surface synthesis despite being crucial in organic chemistry. In this work, we demonstrate how the starting diastereomeric configuration can drive the surface-induced transformation of a substituted cyclooctatetraene (COT) structure—a key molecule in the study of aromaticity—into a cyclopenta[c,d]azulene (CPA) structure, within a chevron-shaped graphene nanoribbon (GNR). Remarkably, the predominant product, the CPA chevron-like GNR, displays the lowest bandgap ever recorded for an all-carbon chevron-shaped GNR. This research opens up new possibilities for applying stereochemical principles in the design of innovative graphene-based nanostructures.
The role of stereochemistry—the spatial arrangement of atoms within molecules—on the reaction pathway has been largely overlooked in on-surface synthesis despite being crucial in organic chemistry. In this work, we demonstrate how the starting diastereomeric configuration can drive the surface-induced transformation of a substituted cyclooctatetraene (COT) structure—a key molecule in the study of aromaticity—into a cyclopenta[c,d]azulene (CPA) structure, within a chevron-shaped graphene nanoribbon (GNR). Remarkably, the predominant product, the CPA chevron-like GNR, displays the lowest bandgap ever recorded for an all-carbon chevron-shaped GNR. This research opens up new possibilities for applying stereochemical principles in the design of innovative graphene-based nanostructures.
Full text in this link
Diastereomeric Configuration Drives an On-Surface Specific Rearrangement into Low Bandgap Non-Benzenoid Graphene Nanoribbons, F. Villalobos, J. I. Mendieta-Moreno, J. Lobo-Checa, S. P. Morcillo, J. I Martínez, J. M. Gómez-Fernández, P. L. de Andres, J. A Martin-Gago, J. M. Cuerva, A. G. Campaña, C. Sánchez-Sánchez, J. Am. Chem. Soc. 147 (2025) 7245.

