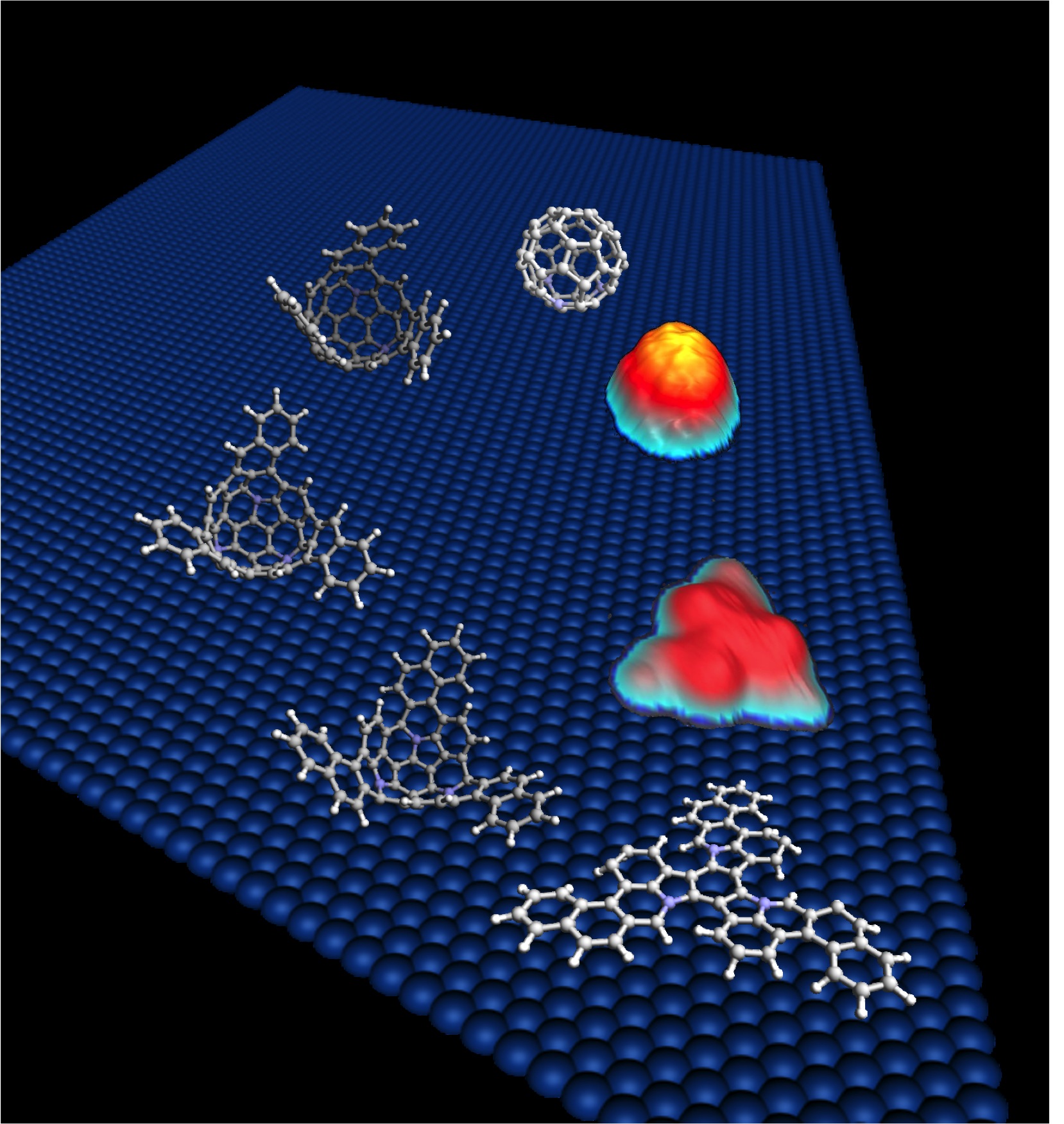
The use of fullerenes in nanotechnology needs an alternative high-controlled mechanism for production of specific molecules. We have found a new process that allows the formation of closed species from planar precursors. The mechanism is a form of molecular “origami”. If you synthesise a planar molecule with the appropriate topology, it can fold into itself to form more complex curved structures. The secret is the use of the catalytic properties of a surface for inducing spontaneous dehydrogenation of the precursor molecule.
The whole process has been visualized by STM (Scanning tunnelling microscopy) the main experimental technique used to follow the process, and the mechanism has been understood by ab-initio calculations.
The advantage of this high efficient process is that if you start with suitable precursors you can obtain heterofullerenes or other different fullerenes in a controlled way. In fact we have made use of this process to synthesise for the first time a heterofullerene C57N3.
Additionally, cyclisation on a controlled flux of different guests could open the door to the encapsulation of atoms or small molecules to form endohedral fullerenes, species not readily available nowadays by other methods in spite of their promising potential applications.
The work has been leaded by Berta Gomez-Lor and Antonio Echavarren for the chemical synthesis, Ruben Pérez for the ab-initio calculations and Jose A. Martín-Gago for the coordination and the experimental part. This work is demonstrating the importance of interdisciplinary collaborations to achieve important goals in the field of nanotechnology.