Many biological molecules present a characteristic chiral structure, which make them suitable for targeting single enantiomer drugs. Also, of basic importance, in t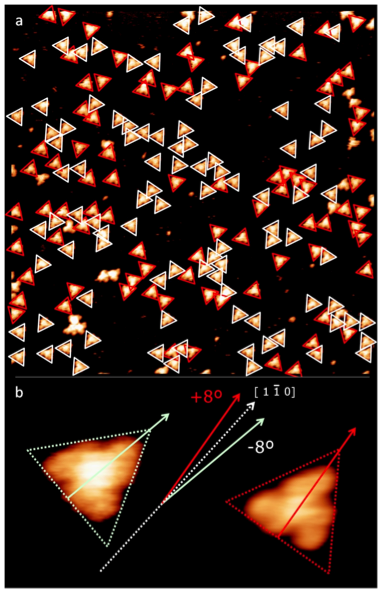 he framework of the origin of life is accepted that a first enantiomeric selection process took place at some stage in the early Earth, originating that exclusively L-amino acids were incorporated into the proteins of living beings. In these two examples the enantiomeric separation is performed either between individual molecules or between a molecule and a surface, without the need of clustering enantiomorphic molecular crystals
he framework of the origin of life is accepted that a first enantiomeric selection process took place at some stage in the early Earth, originating that exclusively L-amino acids were incorporated into the proteins of living beings. In these two examples the enantiomeric separation is performed either between individual molecules or between a molecule and a surface, without the need of clustering enantiomorphic molecular crystals
we show in this letter that thermal under-vacuum deposition on a surface of a three-fold symmetrical polycyclic aromatic hydrocarbon (PAH) leads to an adsorption-induced chirality. This chirality is produced because the molecular landing on the surface could be either on one side or the opposite.
We conclusively demonstrate that the surface «distinguishes» the way in which an individual molecule landed: molecules adsorbed «right» or «left»-hand clearly show different orientations with respect to the underlying substrate. Moreover, we present an atomistic view of the enantiomeric recognition mechanism. We rationalize the interaction of large PAHs on single crystal surfaces concluding that the final configuration maximizes the favorable adsorption of its constituent benzene-like subunits .

