In our article published recently in Nanoscale, we focus on the thermal dehydrogenation of n-octane (n-C8H18) on the catalytic Pt(111) surface in ultra-high vacuum. Our results shed light on the mechanisms behind the initial stages of this industrially relevant reaction, paving the way for the design of efficient dehydrogenation catalysts.
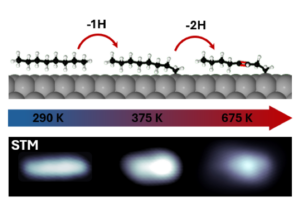
Dehydrogenation of alkanes represents a crucial step for the conversion of these inert sp3 -bonded carbon chains into other valuable unsaturated chemicals. To this end, platinum-based materials are found among the most widely used catalysts. In this work, we characterize this reaction with a combination of surface-sensitive techniques, such as synchrotron-radiation X-ray photoelectron spectroscopy, thermal-programmed desorption and scanning tunnelling microscopy, and ab initio calculations. At low dehydrogenation temperatures, two different dehydrogenation stages are observed. At 330 K, n-C8H18 effectively undergoes a completely regioselective C–H bond cleavage at one of its methyl ends. At 600 K, the chemisorbed molecules suffer a double dehydrogenation, yielding double bonds in their carbon skeletons. Diffusion and clustering of the resulting dehydrogenated species represent the first step towards catalytic poisoning of the surface.
You can find the work published in:
https://doi.org/10.1039/D3NR02564K
In situ observation of the on-surface thermal dehydrogenation of n-octane on Pt(111), Daniel Arribas, Víctor Villalobos-Vilda, Ezequiel Tosi, Paolo Lacovig, Alessandro Baraldi, Luca Bignardi, Silvano Lizzit, José Ignacio Martínez, Pedro Luis de Andres, Alejandro Gutiérrez, José Ángel Martín-Gago and Pablo Merino, Nanoscale 15 (2023) 14458-14467.

