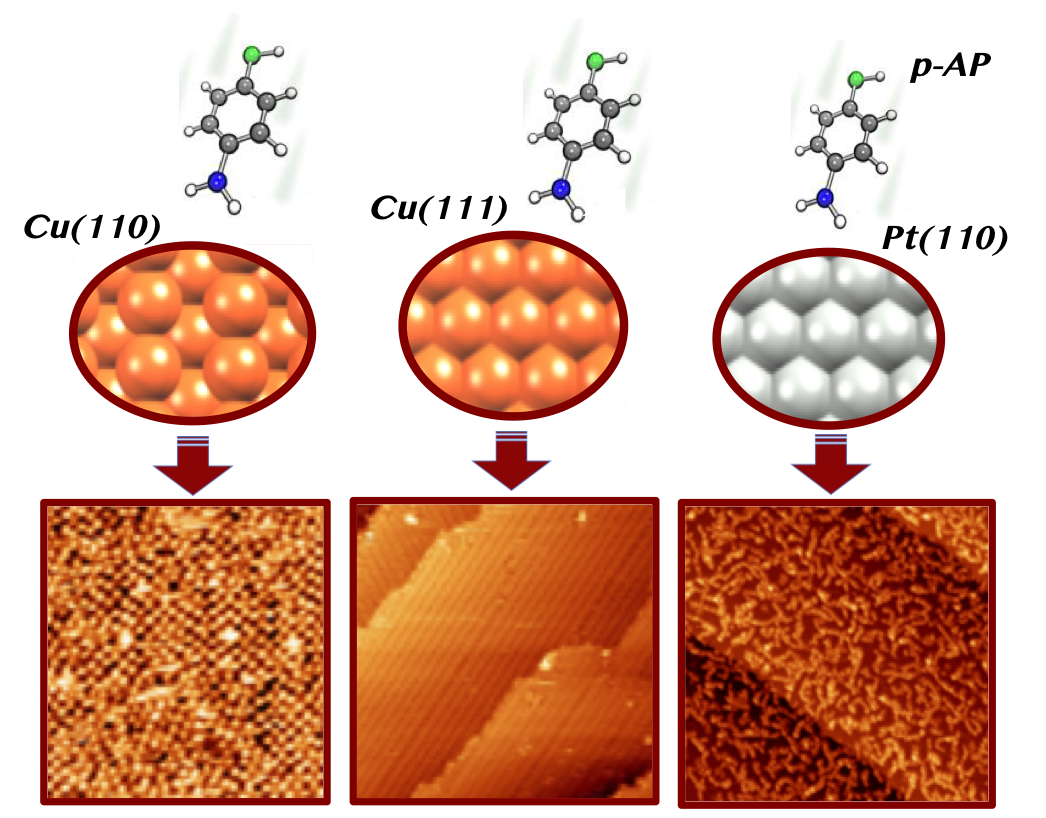
Molecular chemisorption on metal surfaces has attracted great interest over the past decades. Both the characteristics of the adsorbed molecules and the surface properties govern the chemisorption processes. We have studied the role of the nature and symmetry of different metallic surfaces, Cu(110), Cu(111) and Pt(111), on the room-temperature adsorption of p-aminophenol molecules. These interesting molecules consist of a central benzene ring with two functional groups, alcohol and amine in para position. STM, NEXAFS, XPS and LEED experiments have revealed different molecular self-assemblies for the molecules on the different substrates. Thus, the resulting molecular layer presents a specific structural and chemical behaviour depending on the surface. This work shows the greatest influence of surface properties in molecular chemisorption processes.
Full text in this link:

