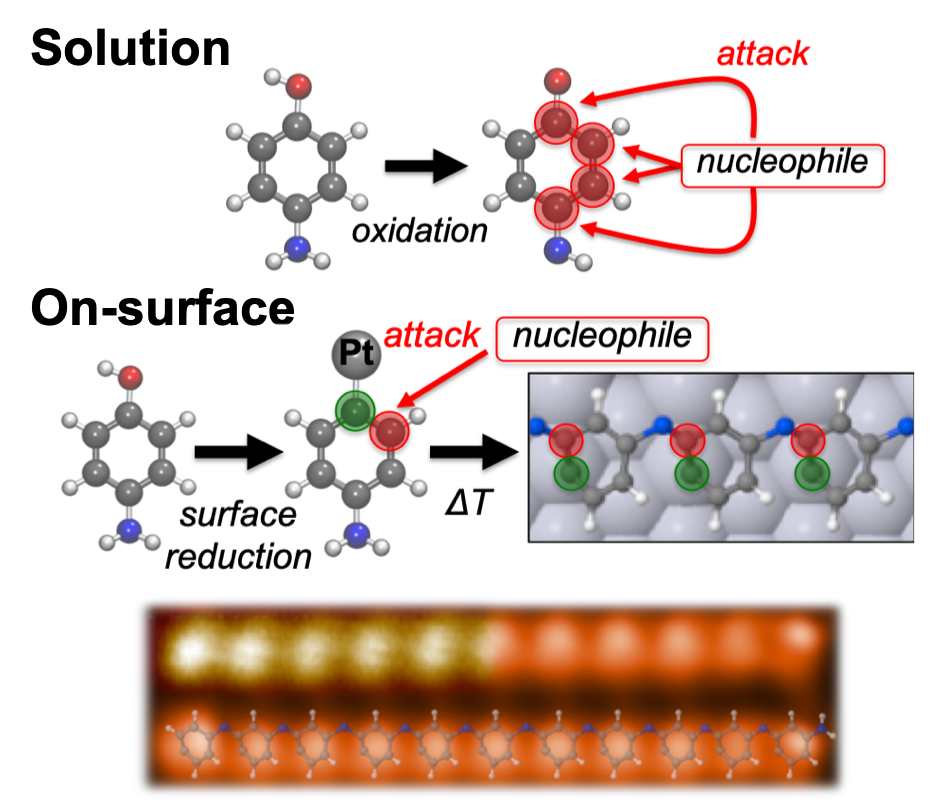
Surfaces never fail to surprise. Once more, they have demonstrated that chemistry on 2D metallic surfaces behaves differently to that on solution (3D). We have shown that highly unspecific solution-based Michael addition transforms into highly selective when carried out on a Pt(111) surface. In this way, we are able to synthesize meta-polyaniline oligomers starting from a para-functionalized precursor, p-aminophenol. This unexpected behavior, which is rationalized by a multitechnique approach including STM, nc-AFM, STS, XPS, and DFT calculations, is composed of 3 steps: dehydrogenation of the alcohol groups, partial dehydrogenation of the amino groups, and intermolecular coupling between activated amino groups and carbon atoms at the meta position. This reaction pathway opens a door towards the highly directional synthesis of novel nanostructures of interest in the emerging fields of nanomagnetic materials and spintronics.
You can find the work published in open access in:

