The contactless heating capacity of magnetic nanoparticles has been exploited in fields such as catalysis, environmental remediation and enzymatic thermal regulation.
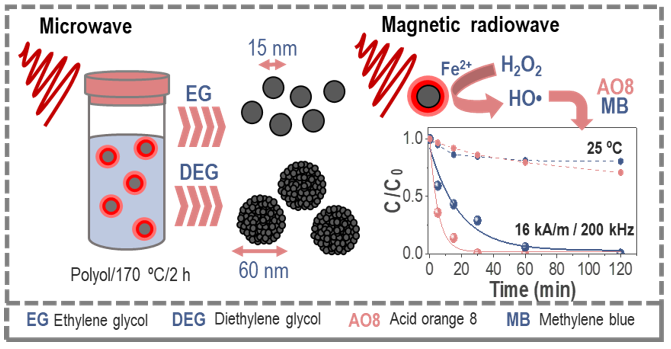
Advanced oxidation processes constitute a promising alternative for the treatment of wastewater containing organic pollutants. Still, the lack of cost-effective processes has hampered the widespread use of these methodologies. Iron oxide magnetic nanoparticles stand as a great alternative since they can be engineered by different reproducible and scalable methods. Multicore nanoparticles showed higher degradation rates and 100% efficiencies in four reusability cycles under the AMF. The improvement of this process with AMF is a step forward into more sustainable remediation techniques [Engineering Iron Oxide Nanocatalysts by a Microwave-Assisted Polyol Method for the Magnetically Induced Degradation of Organic Pollutants].
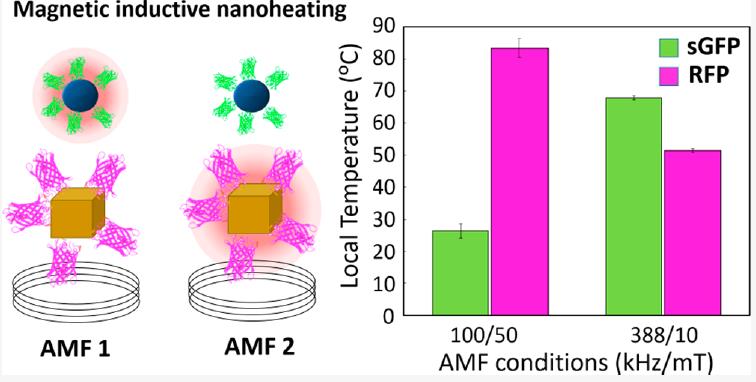
We have proposed an advanced technology to generate multiple local temperatures in a single-pot reactor by exploiting the unique nanoheating features of iron oxide MNPs exposed to alternating magnetic fields (AMFs). The heating power of the MNPs depends on their magnetic features but also on the intensity and frequency conditions of the AMF. Using a mixture of diluted colloids of MNPs we were able to generate a multihot-spot reactor in which each population of MNPs can be selectively activated by adjusting the AMF conditions. The maximum temperature reached at the surface of each MNP was registered using independent fluorescent thermometers that mimic the molecular link between enzymes and MNPs. This technology paves the path for the implementation of a selective regulation of multienzymatic reactions [Selective Magnetic Nanoheating: Combining Iron Oxide Nanoparticles for Multi-Hot-Spot Induction and Sequential Regulation].