On-surface chemistry is a potential strategy focused on obtaining and developing novel nanomaterials of different dimensionalities with atomic precision. The growth mechanism is based on a bottom-up approach where molecular building blocks are evaporated onto a surface. Therefore, choosing the appropriate surface and precursor we are able to build and design new nanomaterials with specific properties.
Concerning this topic we have just published a new work in Angewandte Chemie:
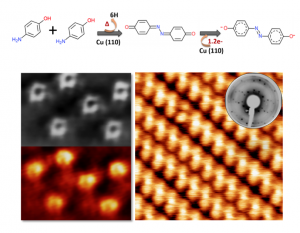
The work presents a promising and novel route for the synthesis of azine derivatives with strong acceptor behavior. In this case, the on-surface synthesis consists on a thermal annealing process where p-aminophenol molecules, deposited on Cu(110), undergo an azine-coupling reaction. Both, the chemical reaction mechanism and the charge transfer process induced by the substrate were followed by complementary surface techniques (nc-AFM/STM, XPS, NEXAFS and LEED) as well as by theoretical calculations. We observe that Cu(110) catalyzes a chemical reaction between two p-Ap molecules giving rise to a quinoneazine molecule. The resulting molecule accepts 1.2e- from the substrate, which induces a charge redistribution with recovering of aromaticity, leading to an azo compound behaviour.
The study opens the door for the use of on-surface synthesis protocols to fabricate à la carte donor or acceptor interfaces on organic heterostructures.

